Bases Water from equilibrium 1 and ammonia from equilibrium 2. Conjugate base of HPO24.

Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com
Conjugate acid of HSO4.

. NH 4 Upvote. Conjugate acid of PO34. Conjugate acid of NH3.
Identify the Conjugate acid-base pairs in the given formula. Up to 25 cash back Identify the conjugate acid for each base. Conjugate base formed by release of H ion and conjugate.
H2CO3 H 2 CO 3 conjugate acid of HPO24 HPO 4 2. Identify each substance as an acid or a base and write a chemical equation showing how it is an acid or a base according to the Arrhenius definition. The conjugate acid-base pairs are HFF and d HCHO2CHO 2.
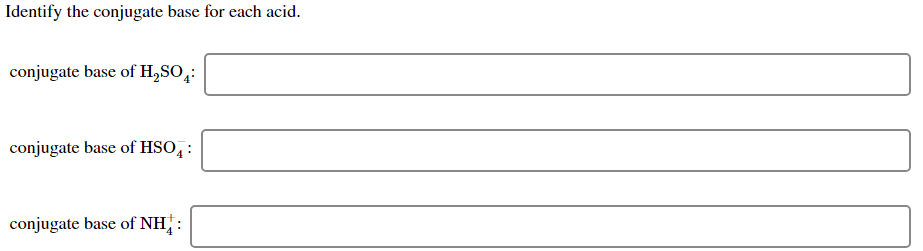
PH for positive by the basis PH Too negative. Conjugate base of Hzsoy Hsoe Conjugate base of HCO3 20 0 3 Conjugate base of NHUT NH 3 Explanation- Dtzsoy is the acid because it donates I ion to water. Start studying Identify acid base conjugate acid conjugate base.
-9020 Sapig oaming e. Label each of the following as being a strong acid a weak acid or a species with negligible acidity. Let us take the example of bicarbonate ions reacting with water to create carbonic acid and hydronium ions.
HNO2 CH3NH2 CH3NH3 NO2. Identify the acid base conjugate acid and conjugate base in this reactions HClO4aq H2Ol H3Oaq ClO4aq Explanation Any reaction is which a proton trasfer takes place from one substance to another is an acid-base reactionMore specifially the proton transfer view is know as the bronsted-lowry defincation of acids and base. Base acid Conj A Conj B.
Conjugate acid of HS. HFaq H 2 Ol Faq H 3 Oaq. Conjugate base of H2CO3 conjugate base of HSO4 conj.
Conjugate acid of H2PO4. For the reaction 2H2Og2H2gO2g the equilibrium concentrations were found to be 5. See the answer See the answer done loading.
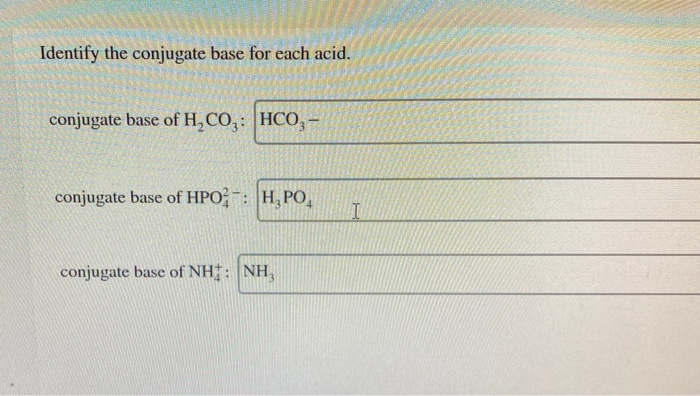
Conjugate base of H2CO3. A and are conjugate acid and base respectively. Identify the conjugate base for each acid.
Congregate Asset is its Threepio for by base comes Out Toby HBO for two negative PS three Conjugated assets. So H₂CO₃ is the conjugate acid of HCO₃. Identify the conjugate base for each acid.
Base They conjugate base of HCOZ 18 103 111 NHy. A conjugate base contains one less H atom and one more - charge than the acid that formed it. Conjugate base of HCO.
Identify the conjugate acid for each base. It has one less H atom and one more charge. Up to 24 cash back base acid conj.
HCO₃ H₂O H₂CO₃ OH. Conjugate base of H2SO conjugate base of HSO4. Conjugate acid of PO34.
It then becomes Hsop which would Hzsoy to - HSOY Hoot Aud conjugate base be conjugate base of Hzsoy H CO2 tho 20 Hot Aud Conjugute. Identify the conjugate base for each acid. Conjugate acid of NH 3.
In 2nd equilibrium NH₄ is the conjugate acid and OH the conjugate base. For each to its congregate as it is etched Rias positive conjugal basis and just negative for each to be awful. We see that HCO₃ becomes H₂CO₃.
Acids H₂CO₃ from equilibrium 1 and water from equilibrium 2. Acids and Bases pH and pOH Acids and Bases 2--How to identify an Acid or Base Calculating pH pOH H H3O OH- of Acids and Bases - Practice AcidBase Dissociation Constant 171c Finding the conjugate of an acid or base. Conjugate base of NH.
Identify the conjugate acid for each base. View the full answer. The conjugate acid-base pairs are H2CO3HCO3 and H3OH2O.
It has one more H atom and one more charge -1 1 0. 112 When ammonium chloride dissolves in water the ammonium ion NH 4 acts as an acid. HCO₃ H₂O H₂CO₃ OH base acid Conj A Conj B.
Conjugate base of H S. The conjugate base of any BL acid is simply that acid less a proton H. Identify the conjugate acid for each base.
- Answered by a verified Tutor We use cookies to give you the best possible experience on our website. Conjugate acid of PO. Conjugate acid of HCO3.
The H₂O becomes OH. Conjugate base is NH 3. According to the Bronsted Lowry concept Bronsted Lowry-acid is a substance that donates one or more hydrogen ion in a reaction and Bronsted Lowry-base is a substance that accepts one or more hydrogen ion in a reaction.
We see that HCO₃ becomes H₂CO₃. Identify the conjugate base for each acid. It has one more H atom and one more charge -1 1 0.
Conjugate base is HSO 4-H 2 PO 4-. Conjugate base is HPO 4 2-NH 4. In 1st equilibrium H₃O is the conjugate acid and HCO₃ the conjugate base.
H2O NH3 NH OH- H20 NH3 NH4 OH- Acid Base Conjugate Acid Conjgate Base Posted 4 days ago. Write the balanced chemical equation for the reaction of the weak acid HCN with water. B and are conjugate acid and base respectively.
Conjugate acid of NH3. Conjugate base of NH. HIaq H 2 Ol Iaq H 3 Oaq Bronsted-Lowry and Arrhenius acid b.
Conjugate acid of NH3. HFaq HSO 3aq Faq H 2 SO 3 aq acid base conj. Identify the conjugate acid for each base.
Conjugate acid of SO24. H 2 SO 4. Learn vocabulary terms and more with flashcards games and other study tools.
Give the formula of the conjugate base of HSO4. So OH is the conjugate base of H₂O. In each case write the formula of its conjugate base and indicate whether the conjugate base is a strong base a weak base or a species with negligible basicity.
A HCOOH b H2 c CH4 d HF e NH4. NH3conjugate base H2Oconjugate acid -- NH4acid OH-base arrow_forward Label the acid base conjugate acid and conjugate base in the following reaction. Asset is EJ to us by bass turns out to be Its too negative.
Or we can say. It just negative as it is its us. HNO 2 aq HSaq NO 2aq H 2 Saq acid base conj.
The conjugate acid-base pairs are HCNCN and HNO2NO2.
Solved Identify The Conjugate Base For Each Acid Er Chegg Com

Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com
Solved Identify The Conjugate Base For Each Acid Conjugate Chegg Com
0 Comments